ANFF-C supports VividWhite in bringing a novel glaucoma treatment device to market
ANFF-C is excited to announce that VividWhite has been awarded Gate 2 pre-seed funding to aid in the commercialisation of VividFlo, a revolutionary microfluidic medical device that provides a novel approach to glaucoma treatment.
Glaucoma is the leading cause of irreversible blindness worldwide, and the second most common cause in Australia. Current treatments for glaucoma, including medication and surgery, often cause side effects and give unpredictable results.
When ophthalmologist, Prof Michael Coote, first envisioned implants for his glaucoma patients to regulate pressure in the eye through controlling fluid movement, he turned to the 3D bio-printing capabilities of ANFF-Materials and UoW’s ARC Centre of Excellence for Electromaterials Science to make his vision a reality. This collaboration paved the way for the technology that is now the basis of this innovative device to stop glaucoma in its tracks.
Formed in 2017, VividWhite is a private Australian, clinical stage, medical device company designing, engineering and manufacturing novel products to treat ophthalmic conditions.
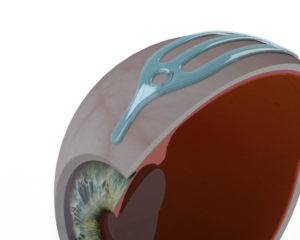
VividWhite’s lead medical device, now in human clinical trials, is VividFlo: a microfluidic surgical implant that relieves intra-ocular pressure by creating a new pathway for fluid drainage from the front of the eye out to surrounding tissue. This innovative device uses advanced manufacturing techniques and precision microfluidics to prevent permanent vision loss from glaucoma.
ANFF-C is a pre-seed fund that uses both its financial and human resources to enable ANFF-derived technologies, such as VividFlo, to realise their commercial potential. We support the launch of new businesses and empower them to attract capital from professional investors by decreasing the risks associated with early-stage entities. Gate 2 supports early-stage projects with funding up to $120,000.

‘VividWhite was delighted to receive ANFF-C funding support to assist our company in developing and manufacturing the novel micro size VividFlo device using nanofabrication technology,’ said Chris Smith, Chief Technology Officer & Biomedical Engineer at VividWhite.
ANFF-C funding will be used to develop tooling to support manufacture of the device as well as surgical guides that will support the implantation of the device. The funding will also help to develop packaging solutions for the distribution of the device.
In December 2023, VividWhite enrolled its first participant into the pivotal registration study for VividFlo. The company’s focus now is on completing participant recruitment for the multi-centre study.
ANFF and ANFF-C are proud to be enabling the continuing journey of VividFlo from conception through commercialisation, helping to save sight one eye at a time through this microscale plumbing system.

