Dr Ann-Na Cho’s hBMS system harnesses the power of microfluidics to create realistic brain organoids to unlock the secrets of childhood dementia.
Dementia doesn’t just affect adults; children and teenagers can develop it too. Caused by 145 different genetic disorders, each child’s experience of this debilitating condition is unique. Individually the genetic disorders are rare, but in Australia, over 100 babies are born with childhood dementia annually, and 91 children die from it each year, a number comparable to childhood cancer.
Sadly, without more research and medical breakthroughs, 70% of children with dementia will continue to lose their lives before they turn 18.

The human brain, with its complex mix of emotions, cognition and consciousness, remains one of the most intricate and poorly understood systems in science. Despite advances, scientists have struggled to fully unravel its mysteries. A significant challenge in translating research into clinical practice is the lack of laboratory models that accurately replicate the brain’s physiological environment.
Brain organoids, miniature brains grown from human stem cells, are valuable for studying how the human brain develops and for researching brain diseases because they mimic actual brain microphysiology.
Organoids can be used to understand brain mechanisms and diseases and test the effects of new therapeutics without the need for animal models.
However, current methods for growing these organoids need improvement to ensure consistent and high-quality results.
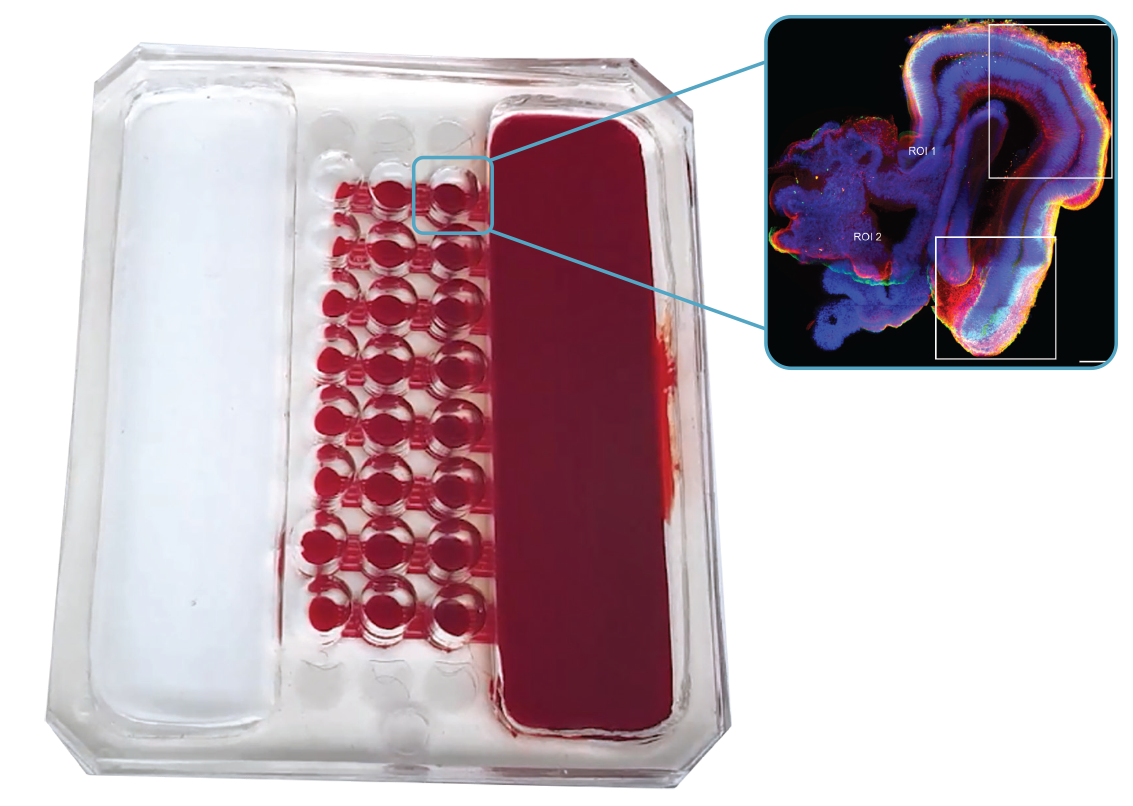
Dr Ann-Na Cho from the University of Sydney is addressing these challenges by creating innovative human brain organoids using advanced biomedical engineering tools. Dr Cho’s approach involves developing a comprehensive system that not only includes the cellular components of the brain but also incorporates surrounding systems such as blood vessels and immune responses, which are crucial for studying brain diseases.
Her ‘Human Brain Microphysiological System’ (hBMS) is a groundbreaking organoid model system built on 2 key components: a human brain extracellular matrix (BEM) to provide the specific signals needed for brain development and a microfluidic device that creates a periodic flow of nutrients to improve the survival and consistency of the organoids.
In essence, Dr Cho’s hBMS creates a more realistic biological environment for the organoids, guiding the development of brain cells more effectively than traditional methods.
By providing brain-specific signals and improving nutrient and oxygen exchange, the system supports cell growth, neuronal differentiation and functional maturation and more accurately replicates actual human embryonic brain development.
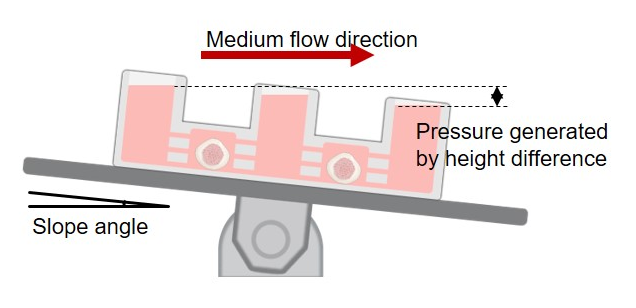
In the hBMS, the microfluidic device plays a crucial role by creating a gravity-driven flow that mimics cerebrospinal and interstitial fluid dynamics. This flow optimises nutrient and oxygen exchange, significantly enhancing cell viability and functionality within the organoids.
Dr Cho’s team has collaborated with ANFF-NSW’s Research and Prototype Foundry, including ANFF engineers Nelson Briones and Ethel Ilagan, to develop the microfluidic device. This device uses a common laboratory stirring device to generate fluid flow, improving organoid survival and differentiation capabilities.
The hBMS model closely mimics human brain physiology and pathology, facilitating the creation of a vascularised brain-on-a-chip. Initially, Dr Cho used the platform to investigate neuroinflammation in response to pathogens, and her research will inform new avenues for treating these potentially devastating infections.
Dr Cho’s work is driven by a commitment to making a real impact on public health, particularly in addressing childhood brain diseases and psychiatric disorders that emerge early in life. The hBMS system has the potential to replace traditional animal models with a better and more relevant system and could transform our understanding of a range of brain diseases, including childhood dementia and developmental disorders such as autism.
Published 15 October 2024 in ANFF’s 2024 Casebook ‘ANFF NEXT‘
Posted 14 May 2025
