Dr Anna Waterhouse and her dynamic research team from the University of Sydney (USYD) are developing next generation surface coatings that reduce the risk of blood clots forming on the implantable medical devices used to treat cardiovascular disease.
For people living with heart and blood vessel disorders, implanted medical devices including stents, vascular grafts and ventricular assist devices can improve or extend their lives. An implant’s biocompatibility is determined by the blood-material interface and blood flow conditions.

Of high concern for the long-term success of a medical implant is the risk of thrombosis, the formation of blood clots. Clotting factors that circulate through the circulatory system can adsorb onto implant surfaces, initiating the conversion of fibrinogen precursors into long fibrin strands. These strands act as a net that traps circulating cells and platelets, forming a blood clot. Blood clots can lead to blocked arteries and other complications that result in device failure. In addition, the clots can break off, especially under high blood flow conditions, causing other life-threatening complications like strokes. Blood thinning treatments can help prevent clots but increase bleeding risk in patients.
Dr Anna Waterhouse, head of USYD’s Cardiovascular Medical Devices Group, is investigating how the material properties of blood contacting medical devices influence blood clot formation. Material surface chemistry, hydrophobicity, roughness and charge can determine the dynamics of protein adhesion onto the device which, in turn, dictates the downstream events in thrombosis.
‘By understanding the blood clotting processes on materials better, we can potentially give patients drugs to break down clots or avoid scenarios where blood clots break off from medical devices,’ said Dr Waterhouse. ‘Ideally, we aim to modify the material surface properties of medical devices to significantly reduce clotting risks and reliance on anti-clotting therapies.’
Currently, researchers study material and blood flow effects by constructing flow circuits from plastic tubing and pumps. Because these channel loops require large volumes of blood (30- 40 mL) to operate, the continual recruitment of human volunteers to donate blood is needed to obtain a sufficient blood supply to run experiments.
Keen to develop a microsystem to mimic blood flow conditions inside a medical device that would also allow her team to manipulate material properties for their experiments, Dr Waterhouse contacted the microfluidic experts at ANFF-SA. Her timing was impeccable, with Dr Waterhouse discovering that ANFF-SA’s free Microengineering School could provide her research assistant and students with the innovative fabrication skills her team required to develop their novel microfluidic device.
Dr Waterhouse also learned that ANFF-SA’s expertise in Computational Fluid Dynamic (CFD) modelling could help optimise the design of their microfluidic device prior to fabrication and inform their experimental parameters. The team’s design requires much smaller blood volumes (5 mL) than conventional test systems. In addition, operators can control flow through the device and precisely determine blood flow dynamics within the system, including flow rate and shear rate. Since shear rate is a key factor in clot formation, Dr Waterhouse’s microdevice can mimic the shear conditions of different medical implant scenarios.
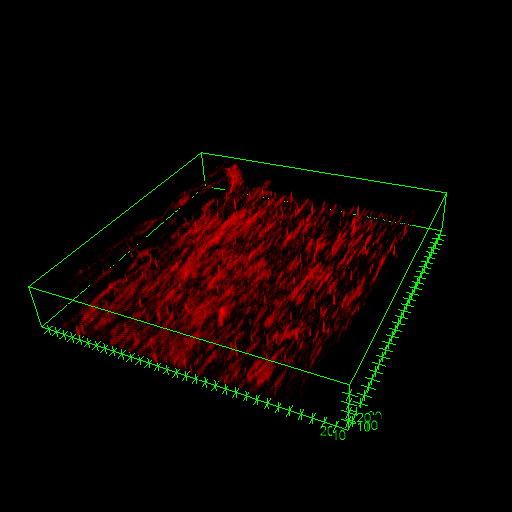
‘With ANFF’s support we have demonstrated that material wettability plays a role in influencing the density of the blood clots which form,’ said Dr Waterhouse. ‘Our research suggests we can modify the material surface properties to modify the clot structure formed, so materials could be made to reduce the risk of clots breaking off and lodging in other organs.’
This is a clear example of the strength of the ANFF network, with the team using the training from ANFF-SA and accessing ANFF-NSW facilities and USYD’s Research and Prototype Foundry to enable Dr Waterhouse’s group to optimise their manufacturing process and rapidly prototype their microfluidic device with access to cutting-edge mask aligners and photolithography equipment.
