Optical-electrode (optrode) technology has emerged as a promising tool that can probe complex electrical signals from cells and tissues.
Neuroscience research has progressed rapidly in the recent past, targeting the treatment and management of many mental conditions and diseases. To study brain physiology, scientists conduct electroencephalogram (EEG) tests in which neural electrical activity is monitored by placing electrodes onto the scalp or into tissues. EEG can be used to determine the overall electrical activity of different brain regions and to diagnose disorders that influence brain activity. However, EEG suffers from limited spatial resolution, providing a rather crude overall picture of brain function.
To gain a more sophisticated understanding of neural activity, researchers need to go deeper into the brain and reduce the size of the electrodes to measure activity at the level of a single neuron. Current technology is insufficient to meet these demands.
Researchers in Scientia Prof. Nigel Lovell’s laboratory at the UNSW Graduate School of Biomedical Engineering and Prof. François Ladouceur’s group at the School of Electrical Engineering and Telecommunications have developed a new probing and monitoring technology based on passively generating optical signals from cellular electrical activity.

PhD candidate Reem Almasri and ANFF-NSW Process Engineer Josiah Firth have fabricated optical electrodes (optrodes) based on a liquid-crystal sensor that can measure bio-electrical signals. Their optrodes passively convert these signals into measurable optical output without using the traditional molecular biology approach of attaching fluorescent tags to biomolecules or cellular structures to convert electrical impulses into an optical output.
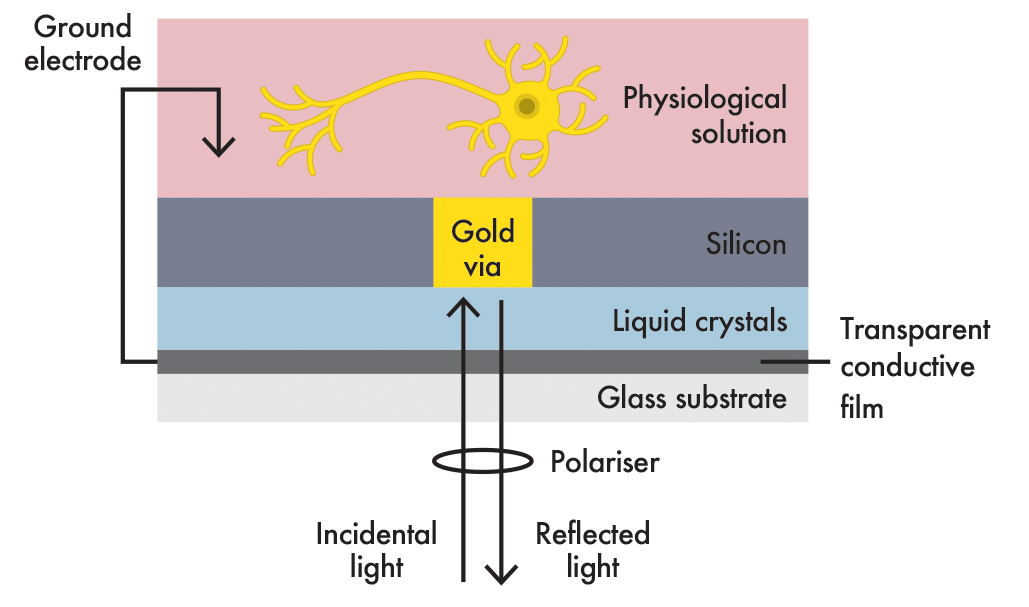
The optrode combines a conductive interface that can detect a biological electrical potential and an underlying layer of liquid crystals that can convert the stimulus into an optical signal. Liquid crystals, not unlike those found in LCD monitors, are very sensitive to electric fields. When a neuron fires, triggering an action potential that changes the electrical field across the cell membrane, there is a corresponding change in the orientation of molecules within the liquid crystal.
An optrode uses an optical fibre to shine light on the liquid crystal sensor and to measure the light reflected from a mirror embedded within the sensor. Neural impulses will induce a measurable change in that reflectance, thus passively transducing the electrical event to an optical signal.
Direct contact of the optrode with soft biological tissues adds some specific requirements on the device properties to achieve a robust, flexible interface. This includes re-engineering biocompatible materials and flexible architectures to transform the rigid industrial version of this sensor into a clinically viable design.
Ms Almasri’s work consists of investigating potential candidate materials and architectures that are compatible with and suitable for the optrode’s design and functionality. In work performed at ANFF-NSW, this re-engineering was achieved by depositing, patterning, and characterising conductive and dielectric flexible materials to mimic the tissue properties and withstand the surrounding biological environment.
This research group’s optrode technology provides a scalable solution that can be employed at the cellular level. They have successfully employed optrodes to record peripheral nerve responses in animal models. This work has expanded beyond neurons to other electrically active cells in the body such as cardiomyocytes, cells in the heart that also produce action potentials. They have successfully used the optrode sensor to record the heart’s electrical signals in animal models.
Dr Saeb Mousavi, a research engineer within the Lovell group, is exploring an alternative optrode fabrication technique that involves direct 3D printing of conductive and insulating inks to achieve flexible optrode devices. This technique is particularly useful as it is simpler and has a lower cost compared to the complicated and expensive cleanroom microfabrication techniques.
Almost one in six people worldwide have a neurological disorder such as dementia, Parkinson’s disease and epilepsy, with cases expected to rise as our populations age. Since these disorders are currently incurable, early diagnosis and monitoring are critical in providing appropriate care that maintains a patient’s quality of life. Given the promise of this innovative optrode technology to provide close monitoring of brain activity, future doctors will have a powerful tool to localise and/or track these neurological disorders, contributing to better patient outcomes.
