Home > News & Events > Creating antibacterial surfaces with smooth edges
Enabled by the MCN and Microscopy Australia, Monash University researchers have engineered new antimicrobial surfaces that can significantly reduce the formation of bacteria on medical instruments and reduce the risk of patient infection while in hospital.
The team have found a potential 3D patterning technique that could prevent the initial formation of microcolonies of Escherichiacoli (E.coli), Klebsiellapneumoniae and Pseudomonas aeruginosa – the three most common urinary tract bacterial infections (UTIs) associated with catheters.
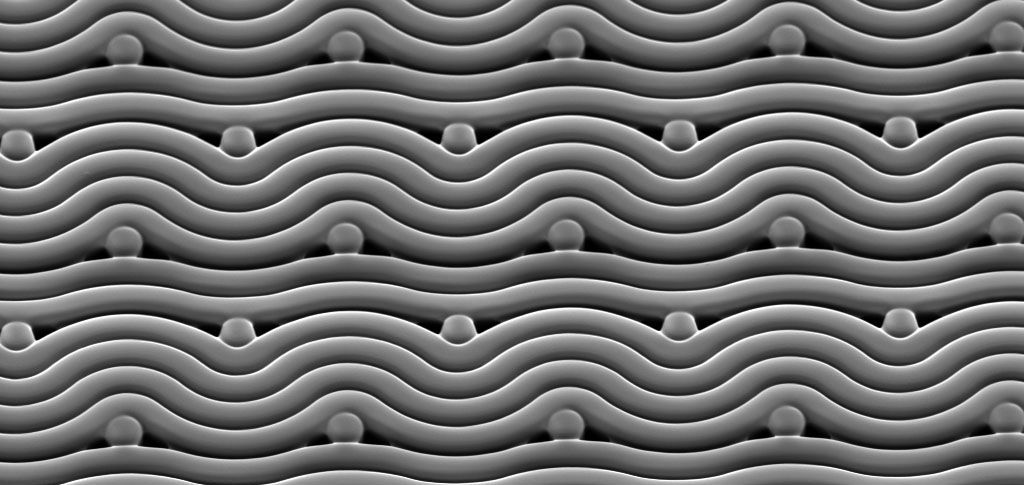
Led by Dr Victor Cadarso from Monash University’s Department of Mechanical and Aerospace Engineering and the Centre to Impact AMR, the researchers have engineered surfaces with smooth micro features instead of the traditional sharp-edged ones to make it more difficult for harmful bacteria to attach to these surfaces in large numbers.
“Using E.coli as an example, we found bacterial cells that form on surfaces do so mostly on the sharp corners. By removing these sharp features, the bacteria can no longer colonise the surface as effectively. This same effect has been demonstrated for the two other pathogens in this study,” said Sara Ghavamian, from the Monash Department of Mechanical and Aerospace Engineering, who created these surfaces.
“Infection control through physically altering the micro architecture of high-touch surfaces within hospitals, such as catheters and ventilators, rather than the traditional use of chemical agents, is not only a more durable approach but also an effective strategy for combating antimicrobial resistance.”
Victor and his team relied heavily on MCN’s photolithography suite, found in the Centre’s Class 100 open access cleanroom space, as they repurposed effects that are typically considered an issue to produce carefully designed smooth novel patterns.
A polymer called “SU-8” is commonly used in the fabrication of microsystems that require vertical sidewalls. This polymer is changed by UV light – it is selectively cured through a mask which is patterned to create a silhouette to only expose parts of the resist that are part of a desired design.
When the SU-8 is exposed to the UV light, it releases a photoacid that creates bonds within the resist and causes it to become less soluble when later developed. If not accounted for, the photoacid can spread throughout the resist, causing issues in most fabrication process flows as nice sharp edges begin to soften and become rounder. However, in this case, this effect is exactly what Victor and his team were after.
Victor explained: “Normally, you will optimize the lithography process to avoid this diffusion of photoacid as it renders defects on the exposure. However, in our work we designed our masks and modified the lithography process to obtain the 3D patterns with high precision, over a large area in a single UV exposure process.”
The standard lithography process includes a post exposure baking step that causes the resist to solidify fully – the time to achieve this solidification is normally minimised to reduce the rate at which the photoacid can travel through the photosensitive material. By carefully controlling this step, the photoacid is allowed to spread and the team were able to encourage their rounded edges and the formation of 3D patterns.
To verify whether their nanofabricated rounded structures performed better than traditional hard-edged features, Sara used Microscopy Australia’s equipment at MCEM and Ramaciotti Centre at Monash University to assess the antimicrobial effects.
While the traditional approach reduced the number of bacterial colonies, it struggled to limit the number of bacteria within those microcolonies that were able to hang on.
Excitingly though, the smooth structures that resulted from the team’s diffused exposure approach were able to demonstrate a simultaneous decrease in both the number of bacterial attachment and microcolony formation compared to the standard flat surfaces.
Sara said: “Developing strategies to prevent the bacterial colonisation of surfaces, such as catheters, without requiring antimicrobial drugs or chemicals is critical to stop biofilm formation and the potential spread of harmful diseases.”
Dr Victor J. Cadarso (Monash University, Department of Mechanical and Aerospace Engineering and Centre to Impact AMR) led the study, performed by Sara Ghavamian (Monash University, Department of Mechanical and Aerospace Engineering and Centre to Impact AMR), with research support from Dr Iain Hay (Monash University, Biomedicine Discovery Institute and School of Biological Sciences, University of Auckland) and Professor Trevor Lithgow (Director, Centre to Impact AMR, and Biomedicine Discovery Institute).
The study was performed in part at the Melbourne Centre for Nanofabrication, as well as the Monash Centre for Electron Microscopy, the Ramaciotti Centre for Cryo-Electron Microscopy, the Monash Centre to Impact AMR and Monash University Biomedicine Discovery Institute.