ANFF-C is excited to announce an IP Licence Option Agreement executed between Monash University and the advanced manufacturing accelerator Innovyz for a nanostructured chip-assisted, drug-screening technology.
Monash University researchers Dr David Rudd and Prof. Nicolas Voelcker have created a new screening platform for the detection of illicit drugs: a nanostructured silicon chip that enables the use of laser technology to drive fast, high throughput testing on-site of a wide array of drugs with a high degree of accuracy.
ANFF-C is excited to announce an IP Licence Option Agreement for this innovative testing platform between Monash University and advancing manufacturing accelerator Innovyz, with a view for an Innovyz-backed company to exclusively licence the technology later in 2023.
A valuable tool for promoting safety, preventing drug abuse and ensuring fairness in a variety of settings, illicit drug screening usually begins with a urine sample. Unfortunately, urine tests sometimes produce false positives. When an initial test is positive, confirmatory laboratory tests are needed to rule out false positives and to identify specific drugs more accurately. So, a sample must be couriered to a pathology lab where a technical expert runs the confirmatory test.
However, confirmatory tests are expensive and time-consuming. Current testing methodology employs a technique called high-performance liquid chromatography – mass spectrometry (HPLC-MS). HPLC separates the components of a mixture based on their chemical and physical properties and their interactions with liquid versus stationary phases in a packed column. The MS detects a component’s molecular weight and then fragments the compound into smaller pieces. Every drug compound has a unique ‘fingerprint-like’ fragmentation pattern related to the chemical’s structure. Laboratory technicians use the sorted mass spectrum to identify and quantify the amount of each component in the sample, providing confirmation of the presence or absence of specific drugs.
HPLC-MS analyses require long processing times because separating the compounds in a mixture takes time, with some longer protocols taking up to 24 hours to complete. HPLC is the rate-limiting step of conventional testing platforms, leading to backlogs in sample testing. The considerable delay between an initial urine screen and the confirmatory laboratory tests comes at high cost. Workers and athletes are sidelined and under suspicion. Employers not only have to pay for additional testing but also lose labour during the wait for a confirmatory result. In the healthcare and criminal justice systems, this delay makes it difficult for medical providers and correctional services to act on information in a timely manner.
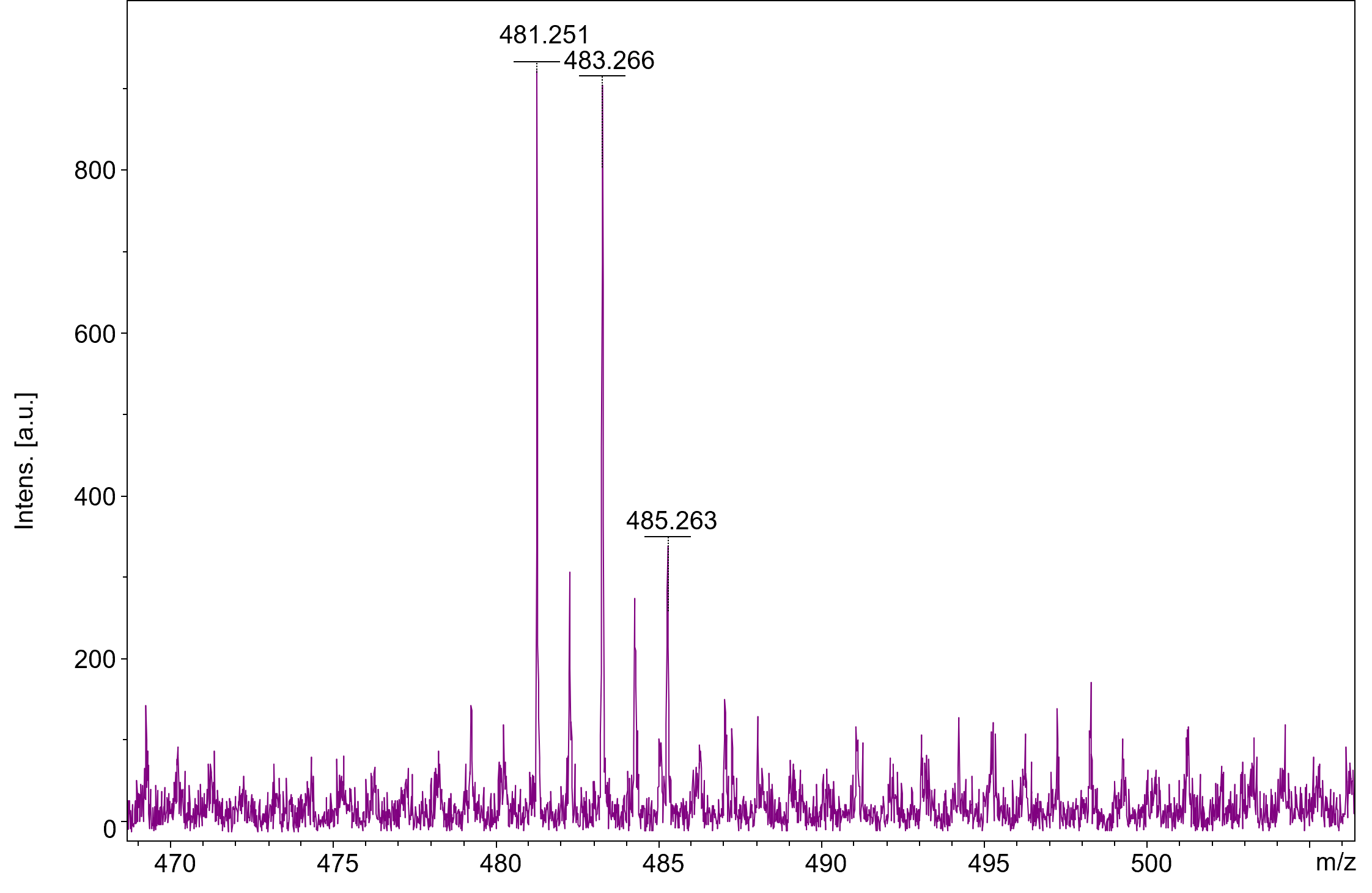
An alternative MS-coupled instrument is matrix-assisted laser desorption/ionisation mass spectrometry (MALDI-MS). Like HPLC-MS, it measures molecular weight and analyses fragment patterns to get a fingerprint for identification. However, the instrument uses lasers fired at a sample mixed with a laser-absorbing chemical (called a matrix). The laser vaporises the matrix and sample, fragmenting all compounds in the sample simultaneously.
MALDI-MS is fast, gathering data in seconds, and simply mixing a sample with a laser-absorbing matrix makes sample preparation fast too. However, although MALDI-MS is widely used in research laboratories to analyse large biomolecules, the method is not suitable for a drug detection platform. The problem is one of molecular mass. The laser vaporises the matrix material into very small fragments. The matrix fragment pattern considerably overlaps and suppresses the molecular weight range where drugs are found. As a result, MS signals created from the presence of drugs in the sample are lost in the noise created by the signals generated from the matrix.
Dr Rudd and Prof. Voelcker have developed a nano-sized solution to the matrix problem. Their innovative technology consists of a nanostructured chip that can be used in combination with a MALDI-MS instrument to detect the presence of small illicit drugs and their metabolites in small volumes of body fluids in a short time.
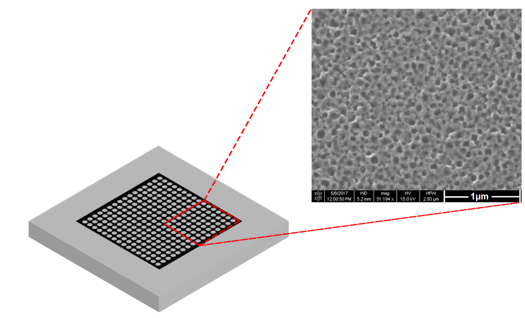
Fabricated at the Melbourne Centre for Nanofabrication (MCN), the chip consists of a nanostructured porous silicon (pSi) film coated with a semi-conducting material. When in contact with a small volume of body fluid, the sample components are retained on the chip’s surface.
The pSi film eliminates the need to mix the sample into matrix material. Inside the MALDI instrument, the chip’s nanotopography optimises vaporisation when the laser is fired at the surface, ejecting the analytes into an ion plume for introduction into the MS.
When using this nanostructured chip, a matrix is no longer needed. The resulting elimination of matrix background noise allows easy detection and identification of small-sized analytes such as amphetamine and cocaine. Their chip can be used to detect multiple drugs in a single analysis from a wide variety of sample types including urine, saliva, blood and breath. The chip surface retains the major illicit drug classes and is adaptable to new illicit drugs. Sample preparation and instrument operation do not require the same level of technical expertise as current practice, enabling testing to be performed by employees in workplaces, treatment clinics, and correctional facilities.
Through discussions with Dr Rudd and Prof. Voelcker, ANFF-C recognised the significant commercial potential of this technology. Since that time, ANFF-C has collaborated with Monash University to accelerate the development of this innovation towards the market. This support included business model analysis and reaching out to several prospective industries and capital parties for feedback early on, which led to discussions with Innovyz, an advanced manufacturing accelerator.
”This new technology possesses the qualities we strive for – the potential to transform industries through commercialisation. Specifically, it could revolutionize law enforcement, healthcare, and workplace safety,” says Derrick Lobban, CEO Innovyz.
Innovyz have since established the company Drugsens Pty Ltd for the technology and have appointed Aditi Dhawan as project manager focused with a view to taking this opportunity forward. In addition to facilitating the Innovyz partnership, ANFF-C and Monash also supported discussions with NSW Correctional Services. A field trial is currently being planned with NSW Correctional Services, with technical project leader Dr David Rudd pleasingly being an ARC Early Career Industry fellowship recipient to advance the platform.
This project is heading for success through the close cooperation of the people who have solved a real problem and those who can support that solution to get to market. It is a clear example of how many players are needed to shape the landscape that can ensure innovative solutions are made into innovative products.
